How Does The Rotavapor Work?
What is a rotavapor?
A 50 liter rotary evaporator (sometimes abbreviated as a rotavap) is a tool primarily used to take away solvent from a pattern by "evaporation beneath decreased strain". The presence of reduced pressure within the tools triggered the solvent (in the spherical bottom flask) to boil at a decrease temperature than normal. Note that rotating the spherical backside flask increases the surface area of the liquid, which will increase the speed of evaporation. The solvent vapor enters the cooler water condenser, where it condenses and drips into a separate receiver bottle. This process is one that removes the solvent, thus leaving the concentrated compound in the unique spherical backside flask.
During routine laboratory operations, it is common to dissolve the desired compound in a solvent. Solvents are used for separatory funnel extraction and column chromatography and have to be eliminated to isolate the desired compound. Solvents with boiling factors decrease than the goal compound are frequently selected, so there is some mechanism to remove them. In principle, it's possible to simply put the solution on a warmth supply to convey the lower boiling solvent to a boil, but this technique is not typically used.
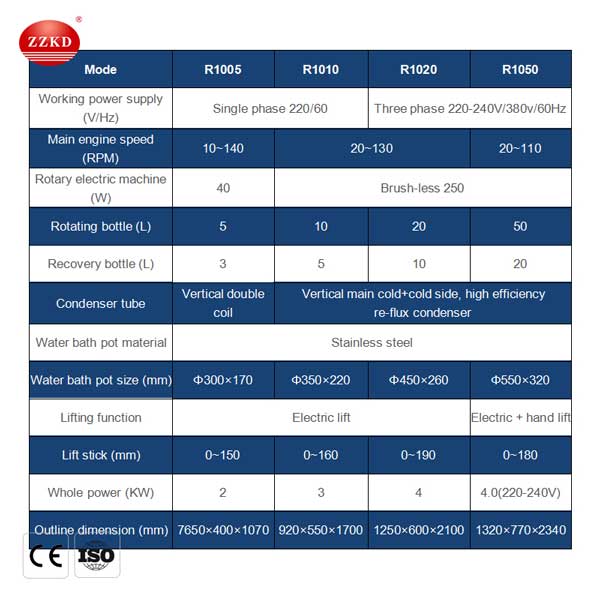
The most well-liked methodology of solvent removal within the laboratory is to use a working principle of rotary evaporator 50l for sale, also referred to as a "rotavap". A rotavapor is basically a vacuum distillation: the solution in a round-bottomed flask is placed in the water bathtub of the device and spun whereas the system is partially evacuated (via a water aspirator or vacuum pump). The decreased pressure within the equipment boils the solvent at a lower temperature than regular (see vacuum distillation), and rotating the flask will increase the surface space of the liquid, which increases the rate of evaporation. The solvent vapor condenses on contact with the water condenser and drips into the receiving bottle. After removal of the solvent, the concentrated compound remained in the flask.

Why use a rotavapor?
Rotary evaporation is usually used to take away relatively low boiling solvents corresponding to EtOAc (ethyl acetate) and n-hexane from samples. This is because working principle of rotavapor are simple to make use of, take away solvent relatively quickly (depending on quantity and solvent), and are present in most natural laboratories. It is also far more efficient than evaporation at atmospheric stress.
However, solvents with higher boiling factors such as water or DMF (dimethylformamide) are tough to take away with commonplace rotavapors and require a vacuum system capable of achieving sufficiently low pressures. For this cause, water is often removed by using a desiccant similar to MgSO4 (magnesium sulfate) before placing the sample in a working principle of rotavapor.
How to make use of it?
Working principle of rotavapor are comparatively simple to make use of, but correct procedures should be adopted.
●Turn on the water bathtub and set to the relevant temperature.
●Make positive the water follows into the water condenser.
●Connect the round backside flask to the rotavapor. Remember to use clips to verify the flask does not slip off!
●Turn on the vacuum pump, then immediately shut the tap to depressurize the system.
●Turn on rotation.
●Before lowering the round-bottom flask into the water tub, wait a second for a collision to occur.
●The spherical bottom flask was monitored till the solvent was eliminated.
●When full, take away the spherical bottom flask from the water bath and cease spinning.
●Turn off the vacuum pump and open the faucet instantly however fastidiously to launch the system from the depressurized state.
●The spherical bottom flask should now be able to be removed from the rotavapor.
How to begin rotavapor works?
1. Let the hot bathtub get sizzling and the condenser cold. The opposite often produces suboptimal outcomes. Given that previous customers nearly actually did not empty the solvent entice. Be cautious when filling the entice with an unknown solvent, as previous customers virtually all the time crammed it with pyridine or trifluoroacetic acid.
2. Secure the collision entice and vial with clips. Alternatively, see our web page on how to extract 5 mg of advanced intermediates from a few liters of algae-infested onerous water.
3. Start the rotor. It ought to spin quick sufficient to create a good coating on the inside surface of the flask. Use this time to review the consequences of the Coriolis force.
4. Start the vacuum pump. Close the stopcock on the condenser to the point where you can no longer hear it whine, but when you cover it together with your thumb and launch it, you'll hear a "pop". Allow the sample to spin beneath vacuum for roughly one minute. Most doubtless it'll begin boiling soon. Don't panic. Boiling does not equal bumping. Let it boil so lengthy as the air bubbles don't attain the neck of the flask. If the bubble does attain the neck, you've got allowed it to collide: congratulations. If air bubbles seem to be at risk of reaching the neck, repressurize the system by fully opening the stopcock to stop boiling. Repeat until the boiling stops and the solvent flows steadily from the condenser; solely then are you able to utterly close the stopcock. When condensation begins to kind on the outer floor of the flask, place it about midway into the hot bath.
5. Continue to observe the situation for a minute or two. If there's a threat of collision, open the stopcock. Repeat again till the boiling stops and the solvent flows steadily from the condenser. At this level, it is secure to leave the rotavapor unattended.
6.Occasionally examine to ensure there are not any important errors.
How to Stop otavapor works?
1. Remove the flask from the heat bathtub.
2. Open the stopcock.
3. Stop the rotor.
4. Turn off the vacuum/aspirator.
5. Disconnect the flask.
6. Place the flask within the hot bathtub.




